Active Ingredients: Each tablet, caplet, or geltab contains Acetaminophen 250 mg, Aspirin 250 mg, and Caffeine 65 mg.
Inactive Ingredients: (tablet, caplet) benzoic acid, carnauba wax, hydroxypropylcellulose, hydroxypropyl methylcellulose, microcrystalline cellulose, mineral oil, polysorbate 20, povidone, propylene glycol, simethicone emulsion, sorbitan monolaurate, stearic acid.
May also contain: FD&C blue # 1, titanium dioxide
Inactive Ingredients: (geltab) benzoic acid, D&C yellow #10 lake, disodium EDTA, FD&C blue #1 lake, FD&C red # 40 lake, ferric oxide, gelatin, glycerin, hydroxypropylcellulose, hydroxypropyl methylcellulose, maltitol solution, microcrystalline cellulose, mineral oil, pepsin, polysorbate 20, povidone, propylene glycol, propyl gallate, simethicone emulsion, sorbitan monolaurate, stearic acid, titanium dioxide.
Uses: For the temporary relief of minor aches and pains associated with headache, sinusitis, a cold, muscular aches, premenstrual and menstrual cramps, toothache, and for the minor pain from arthritis.
Warnings: Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye' syndrome, a rare but serious illness reported to be associated with aspirin.
Alcohol Warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take acetaminophen and aspirin or other pain relievers/fever reducers. Acetaminophen and aspirin may cause liver damage and stomach bleeding.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product. IT IS ESPECIALLY IMPORTANT NOT TO USE ASPIRIN DURING THE LAST 3 MONTHS OF PREGNANCY UNLESS SPECIFICALLY DIRECTED TO DO SO BY A DOCTOR BECAUSE IT MAY CAUSE PROBLEMS IN THE UNBORN CHILD OR COMPLICATIONS DURING DELIVERY. Do not take this product for pain for more than 10 days or for fever for more than 3 days unless directed by a doctor. If pain or fever persists or gets worse, if new symptoms occur, or if redness or swelling is present, consult a doctor because these could be signs of a serious condition. Consult a dentist promptly for toothache. Do not take this product if you are allergic to aspirin, have asthma, have stomach problems (such as heartburn, upset stomach or stomach pain) that persist or recur, or if you have ulcers or bleeding problems, unless directed by a doctor. If ringing in the ears or loss of hearing occurs, consult a doctor before taking any more of this product.
Drug Interaction Precaution: Do not take this product if you are taking a prescription drug for anticoagulation (thinning the blood), diabetes, gout or arthritis unless directed by a doctor.
Directions: Adults: 2 tablets, caplets or geltabs with water every 6 hours while symptoms persist, not to exceed 8 tablets, caplets or geltabs in 24 hours, or as directed by a doctor. Children under 12 years of age: Consult a doctor.
Overdose: Acetylcysteine As An Antidote For Acetaminophen Overdose
Acetaminophen is rapidly absorbed from the upper gastrointestinal tract with peak plasma levels occurring between 30 and 60 minutes after therapeutic doses and usually within 4 hours following an overdose. The parent compound, which is nontoxic, is extensively metabolized in the liver to form principally the sulfate and glucuronide conjugates which are also nontoxic and are rapidly excreted in the urine. A small fraction of an ingested dose is metabolized in the liver by the cytochrome P-450 mixed function oxidase enzyme system to form a reactive, potentially toxic, intermediate metabolite which preferentially conjugates with hepatic glutathione to form the nontoxic cysteine and mercapturic acid derivatives which are then excreted by the kidney. Therapeutic doses of acetaminophen do not saturate the glucuronide and sulfate conjugation pathways and do not result in the formation of sufficient reactive metabolite to deplete glutathione stores. However, following ingestion of a large overdose (150 mg/kg or greater) the glucuronide and sulfate conjugation pathways are saturated resulting in a larger fraction of the drug being metabolized via the P-450 pathway. The increased formation of reactive metabolite may deplete the hepatic stores of glutathione with subsequent binding of the metabolite to protein molecules within the hepatocyte resulting in cellular necrosis. Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours postingestion. In most adults and adolescents, regardless of the quantity of acetaminophen reported to have been ingested, administer acetylcysteine immediately. Acetylcysteine therapy should be initiated and continued for a full course of therapy. Its effectiveness depends on early administration, with benefit seen principally in patients treated within 16 hours of the overdose.
If acetaminophen plasma assay capability is not available, and the estimated acetaminophen ingestion exceeds 150 mg/kg, acetylcysteine therapy should be initiated and continued for a full course of therapy.
For full prescribing information, refer to the acetylcysteine package insert. Do not await the results of assays for acetaminophen level before initiating treatment with acetylcysteine. The following additional procedures are recommended: The stomach should be emptied promptly by lavage or by induction of emesis with syrup of ipecac. A serum acetaminophen assay should be obtained as early as possible, but no sooner than four hours following ingestion. Liver function studies should be obtained initially and repeated at 24-hour intervals.
For additional emergency information call your regional poison center or toll-free (1-800-525-6115) to the Rocky Mountain Poison Center for assistance in diagnosis and for directions in the use of acetylcysteine as an antidote.
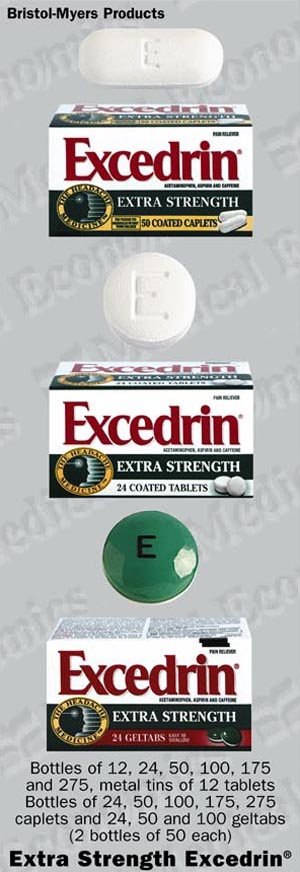
How Supplied: Extra Strength EXCEDRIN® is supplied as:
Coated white circular tablet with letter "E" debossed on one side. Supplied in bottles of 12's, 24's, 50's, 100's, 175's, 275's and metal tins of 12's.
Coated white caplets with "E" debossed on one side. Supplied in bottles of 24's, 50's, 100's, 175's, 275's.
Gel-coated round geltabs-green on one side, white on the other, printed with black "E" on one side. Supplied in bottles of 24's, 50's and 100's (2 bottles of 50 each).
Store at room temperature.
 |